Thursday, February 10, 2011
Friday, July 10, 2009
Thursday, July 9, 2009
HALOGENS
HALOGENS ON THE RIGHT
Halogens on the Periodic Table In the second column from the right side of the periodic table, you will find Group Seventeen (Group XVII). This column is the home of the halogen family of elements. Who is in this family? The elements included are Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
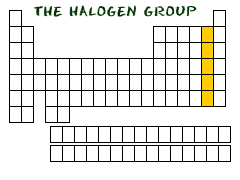
WHAT MAKES THEM SIMILAR?
When you look at our descriptions of the elements fluorine (F) and chlorine (Cl) you will see that they both have seven electrons in their outer shell. That seven-electron idea applies to all of the halogens. They are all just one electron shy of having full shells. Because they are so close to being happy, they have the trait of combining with many different elements. You will often find them bonding with metals and elements from Group One of the periodic table.
Sodium Chloride is a Halogen We've just told you how reactive they are. Not all halogens react with the same intensity. Fluorine is actually the most reactive and combines all of the time. As you move down the column, reactivity decreases. As you learn more about the table, you will find this pattern true for other families.
THEN WHAT IS A HALIDE?
The elements we are talking about in this section are called halogens. When a halogen combines with another element, the resulting compound is called a halide. One of the best examples of a halide is sodium chloride (NaCl). Don't think that the halogens always make ionic compounds. Many halides of the world are made with covalent compounds.
Halogens on the Periodic Table In the second column from the right side of the periodic table, you will find Group Seventeen (Group XVII). This column is the home of the halogen family of elements. Who is in this family? The elements included are Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
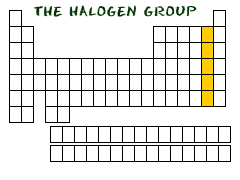
WHAT MAKES THEM SIMILAR?
When you look at our descriptions of the elements fluorine (F) and chlorine (Cl) you will see that they both have seven electrons in their outer shell. That seven-electron idea applies to all of the halogens. They are all just one electron shy of having full shells. Because they are so close to being happy, they have the trait of combining with many different elements. You will often find them bonding with metals and elements from Group One of the periodic table.
Sodium Chloride is a Halogen We've just told you how reactive they are. Not all halogens react with the same intensity. Fluorine is actually the most reactive and combines all of the time. As you move down the column, reactivity decreases. As you learn more about the table, you will find this pattern true for other families.
THEN WHAT IS A HALIDE?
The elements we are talking about in this section are called halogens. When a halogen combines with another element, the resulting compound is called a halide. One of the best examples of a halide is sodium chloride (NaCl). Don't think that the halogens always make ionic compounds. Many halides of the world are made with covalent compounds.
Transition metals
TRANSITIONING
Transition metals in the periodic table Lets start off by telling you that there are a lot of elements that are considered transition metals. Which metals are the transition metals?
21 (Scandium) through 29 (Copper)
39 (Yttrium) through 47 (Silver)
57 (Lanthanum) through 79 (Gold)
89 (Actinium) and all higher numbers.

WHAT MAKES THEM SO SPECIAL?
It all has to do with their shells/orbitals. In CHEM4KIDS we try to stick to the first 18 elements because they are easy to explain. Transition metals are good examples of advanced shell ideas. They have a lot of electrons and distribute them in different ways.
Number of electrons allowed in the orbitals of transition metals. Transition metals are able to put more than eight electrons in the shell that is one in from the outermost shell. Think about argon (Ar). It has 18 electrons set up in a 2-8-8 order. Scandium is only 3 spots away with 21 electrons, but it has a configuration of 2-8-9-2. Wow! This is where it starts. This is the point in the periodic table where you can place more than 8 electrons in a shell.

The transition metals are able to put up to 32 electrons in their second to last shell. Something like gold (Au) has an organization of 2-8-18-32-18-1. Of course, there are still some rules. No shell can have more than 32 electrons. It's usually 18 or 32 for the maximum number of electrons.
ONE MORE THING
Most elements can only use electrons from their outer orbital to bond with other elements. Transition metals can use the two outermost shells/orbitals to bond with other elements. It's a chemical trait that allows them to bond with many elements in a variety of shapes. Why can they do that?
As you learn more, you will discover that most transition elements actually have two shells that are not happy. Whenever you have a shell that is not happy, its electrons can bond with other elements. Example: Molybdenum (Mo) with 42 electrons. The configuration is 2-8-18-13-1. The shells with 13 and 1 are not happy. Those two orbitals can use the electrons to bond with other atoms.
Transition metals in the periodic table Lets start off by telling you that there are a lot of elements that are considered transition metals. Which metals are the transition metals?
21 (Scandium) through 29 (Copper)
39 (Yttrium) through 47 (Silver)
57 (Lanthanum) through 79 (Gold)
89 (Actinium) and all higher numbers.

WHAT MAKES THEM SO SPECIAL?
It all has to do with their shells/orbitals. In CHEM4KIDS we try to stick to the first 18 elements because they are easy to explain. Transition metals are good examples of advanced shell ideas. They have a lot of electrons and distribute them in different ways.
Number of electrons allowed in the orbitals of transition metals. Transition metals are able to put more than eight electrons in the shell that is one in from the outermost shell. Think about argon (Ar). It has 18 electrons set up in a 2-8-8 order. Scandium is only 3 spots away with 21 electrons, but it has a configuration of 2-8-9-2. Wow! This is where it starts. This is the point in the periodic table where you can place more than 8 electrons in a shell.

The transition metals are able to put up to 32 electrons in their second to last shell. Something like gold (Au) has an organization of 2-8-18-32-18-1. Of course, there are still some rules. No shell can have more than 32 electrons. It's usually 18 or 32 for the maximum number of electrons.
ONE MORE THING
Most elements can only use electrons from their outer orbital to bond with other elements. Transition metals can use the two outermost shells/orbitals to bond with other elements. It's a chemical trait that allows them to bond with many elements in a variety of shapes. Why can they do that?
As you learn more, you will discover that most transition elements actually have two shells that are not happy. Whenever you have a shell that is not happy, its electrons can bond with other elements. Example: Molybdenum (Mo) with 42 electrons. The configuration is 2-8-18-13-1. The shells with 13 and 1 are not happy. Those two orbitals can use the electrons to bond with other atoms.
Subscribe to:
Posts (Atom)